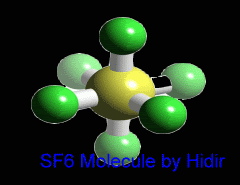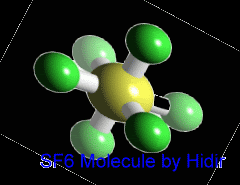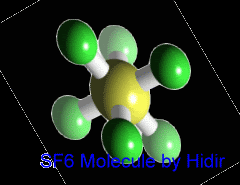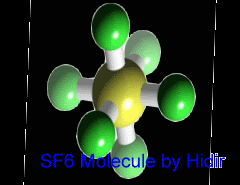
Sulfur hexafluoride (SF6) is an inorganic, colorless, odorless, non-toxic and non-flammable greenhouse gas. SF6 has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. It is a hypervalent molecule. Typical for a nonpolar gas, it is poorly soluble in water but soluble in nonpolar organic solvents. It is generally transported as a liquefied compressed gas. It has a density of 6.12 g/L at sea level conditions, which is considerably higher than the density of air.
SF6 is used in the electrical industry as a gaseous dielectric medium for high-voltage circuit breakers, switchgear, and other electrical equipment, often replacing oil filled circuit breakers (OCBs) that can contain harmful PCBs. SF6 gas under pressure is used as an insulator in gas insulated switchgear (GIS) because it has a much higher dielectric strength than air or dry nitrogen. This property makes it possible to significantly reduce the size of electrical gear. This makes GIS more suitable for certain purposes such as indoor placement, as opposed to air-insulated electrical gear, which takes up considerably more room. Gas-insulated electrical gear is also more resistant to the effects of pollution and climate, as well as being more reliable in long-term operation because of its controlled operating environment.
(Taken from http://en.wikipedia.org/wiki/Sulfur_hexafluoride)
No comments:
Post a Comment